GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: ML-1709460 | Zactran® (veterinary)
Compound class:
Synthetic organic
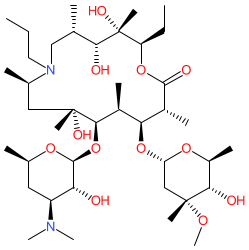
Comment: Gamithromycin is a 15-membered macrolide (azalide subclass) antibacterial compound. It was developed for use in veterinary medicine.
|
|
|||||||||||||||||||||||||||||||||||
Classification  |
|
| Compound class | Synthetic organic |
International Nonproprietary Names  |
|
| INN number | INN |
| 8754 | gamithromycin |
Synonyms  |
| ML-1709460 | Zactran® (veterinary) |
Database Links  |
|
| Specialist databases | |
Antibiotic DB

|
Gamithromycin |
| Other databases | |
| CAS Registry No. | 145435-72-9 (source: Scifinder) |
| ChEBI | CHEBI:195437 |
| ChEMBL Ligand | CHEMBL2107342 |
| DrugBank Ligand | DB11416 |
| DrugCentral Ligand | 5548 |
| GtoPdb PubChem SID | 507750322 |
| PubChem CID | 59364992 |
| Search Google for chemical match using the InChIKey | VWAMTBXLZPEDQO-UZSBJOJWSA-N |
| Search Google for chemicals with the same backbone | VWAMTBXLZPEDQO |
| Search PubMed clinical trials | gamithromycin |
| Search PubMed titles | gamithromycin |
| Search PubMed titles/abstracts | gamithromycin |
| UniChem Compound Search for chemical match using the InChIKey | VWAMTBXLZPEDQO-UZSBJOJWSA-N |
| UniChem Connectivity Search for chemical match using the InChIKey | VWAMTBXLZPEDQO-UZSBJOJWSA-N |