GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: MC-13 [6]
Compound class:
Natural product
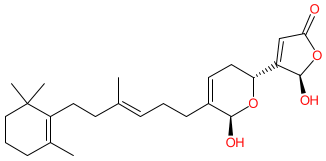
Comment: Manoalide is a sesterterpenoid compound that has been isolated from species of marine sponges such as Luffariella variabilis.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Dal Piaz F, Casapullo A, Randazzo A, Riccio R, Pucci P, Marino G, Gomez-Paloma L. (2002)
Molecular basis of phospholipase A2 inhibition by petrosaspongiolide M. Chembiochem, 3 (7): 664-71. [PMID:12325001] |
|
2. De Rosa S, Crispino A, De Giulio A, Iodice C, Benrezzouk R, Terencio MC, Ferrándiz ML, Alcaraz MJ, Payá M. (1998)
A new cacospongionolide inhibitor of human secretory phospholipase A2 from the Tyrrhenian sponge Fasciospongia cavernosa and absolute configuration of cacospongionolides. J Nat Prod, 61 (7): 931-5. [PMID:9677277] |
|
3. Glaser KB, Jacobs RS. (1986)
Molecular pharmacology of manoalide. Inactivation of bee venom phospholipase A2. Biochem Pharmacol, 35 (3): 449-53. [PMID:3947381] |
|
4. Glaser KB, Jacobs RS. (1987)
Inactivation of bee venom phospholipase A2 by manoalide. A model based on the reactivity of manoalide with amino acids and peptide sequences. Biochem Pharmacol, 36 (13): 2079-86. [PMID:3111475] |
|
5. Glaser KB, Vedvick TS, Jacobs RS. (1988)
Inactivation of phospholipase A2 by manoalide. Localization of the manoalide binding site on bee venom phospholipase A2. Biochem Pharmacol, 37 (19): 3639-46. [PMID:3178877] |
|
6. Iobbi V, Parisi V, Giacomini M, De Riccardis F, Brun P, Nunez-Pons L, Drava G, Giordani P, Monti MC, Poggi R. (2025)
Sesterterpenoids: sources, structural diversity, biological activity, and data management. Nat Prod Rep, Advance Article. DOI: 10.1039/D4NP00041B |