GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Synthetic organic
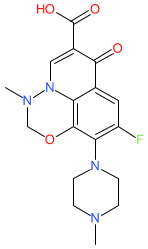
Comment: Marbofloxacin is a fluoroquinolone antibacterial compound, with broad-spectrum activity against both Gram-positive and Gram-negative bacteria [1]. It was developed for use in veterinary medicine.
|
|
|||||||||||||||||||||||||||||||||||