GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: Naxcel® (veterinary)
Compound class:
Synthetic organic
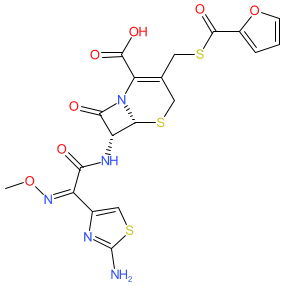
Comment: Ceftiofur is a third generation cephalosporin belonging to the β-lactam class of antibacterial compounds. It has activity against Gram-positive and Gram-negative bacteria and was developed for use in veterinary medicine [1].
|
|
|||||||||||||||||||||||||||||||||||
Download 2D Structure  |
|
| Canonical SMILES | Download |
| Isomeric SMILES | Download |
| InChI standard identifier | Download |
| InChI standard key | Download |
Molecular structure representations generated using Open Babel