GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
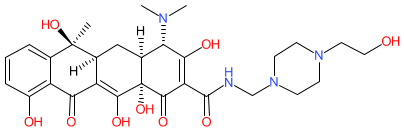
Synonyms: mepicycline
Compound class:
Synthetic organic
Comment: Pipacycline is a tetracycline antibacterial compound. Note that the structure shown here matches the CAS-assigned structure. The PubChem link, provided in the table below, is to their standardized chemical structure (CID 54686184), although this is not an exact structural match.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Penimepicycline, the salt of pipacycline and phenoxymethylpenicillin, was investigated as a treatment for urological, gynecological, and respiratory infections during the 1960s. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Tetracyclines inhibit bacterial protein synthesis by binding reversibly to the bacterial 30S ribosomal subunit and preventing the aminoacyl tRNA from binding to the acceptor (A) site on the mRNA-ribosome complex (reviewed in [1-2]). |