GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
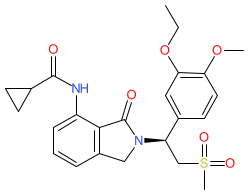
Synonyms: AMG-634 | CC-11050
Compound class:
Synthetic organic
Comment: Dovramilast is a phosphodiesterase 4 (PDE4) inhibitor with anti-inflammatory properties. It is in clinical development as an adjunctive host-directed therapy, to be used in the treatment of leprosy (Hansen's disease) and in combination with conventional antibacterial regimens as a strategy for shortening the duration of treatment for tuberculosis (TB).
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| In November 2023, the US FDA issued Orphan Drug Designation for dovramilast as a treatment for leprosy type 2 reactions (also known as erythema nodosum leprosum), a severe complication of leprosy that is immune-mediated. Dovramilast has completed a Phase 2 clinical trial (NCT01300208) to evaluate safety and efficacy in subjects with leprosy type 2 reactions, with a second trial now recruiting (NCT03807362). It has also completed a Phase 2 study, as an adjunctive host-directed TB therapy (NCT02968927) and found to be safe, reasonably well tolerated, and with potential to enhance recovery [3]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT01300208 | To Evaluate the Preliminary Safety, Tolerability, Pharmacokinetics, Pharmacodynamics and Efficacy of CC-11050 in Subjects With Discoid Lupus Erythematosus and Subacute Cutaneous Lupus Erythematosus | Phase 2 Interventional | Amgen | ||
| NCT03807362 | CC-11050 Trial in Nepalese Patients With Erythema Nodosum Leprosum | Phase 2 Interventional | The Leprosy Mission Nepal | ||
| NCT02968927 | TB Host Directed Therapy | Phase 2 Interventional | The Aurum Institute NPC | 3 | |