GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Synthetic organic
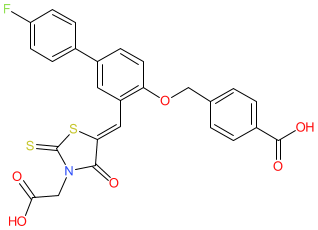
Comment: This small molecule is reported as an inhibitor of NAD(P) dependent 3-beta-hydroxysteroid dehydrogenase NSDHL [5], an enzyme that is essential for cholesterol synthesis. Inhibiting NSDHL is being explored as an anti-tumour mechanism [2-3], since NSDHL activity has also been implicated in epidermal growth factor receptor (EGFR) trafficking pathways [1,4]. Additional evidence supports targeting NSDHL for clinical benefit in cholesterol-related diseases.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Beck TN, Georgopoulos R, Shagisultanova EI, Sarcu D, Handorf EA, Dubyk C, Lango MN, Ridge JA, Astsaturov I, Serebriiskii IG et al.. (2016)
EGFR and RB1 as Dual Biomarkers in HPV-Negative Head and Neck Cancer. Mol Cancer Ther, 15 (10): 2486-2497. [PMID:27507850] |
|
2. Chen M, Zhao Y, Yang X, Zhao Y, Liu Q, Liu Y, Hou Y, Sun H, Jin W. (2021)
NSDHL promotes triple-negative breast cancer metastasis through the TGFβ signaling pathway and cholesterol biosynthesis. Breast Cancer Res Treat, 187 (2): 349-362. [PMID:33864166] |
|
3. Ershov P, Kaluzhskiy L, Mezentsev Y, Yablokov E, Gnedenko O, Ivanov A. (2021)
Enzymes in the Cholesterol Synthesis Pathway: Interactomics in the Cancer Context. Biomedicines, 9 (8). [PMID:34440098] |
|
4. Gabitova L, Restifo D, Gorin A, Manocha K, Handorf E, Yang DH, Cai KQ, Klein-Szanto AJ, Cunningham D, Kratz LE et al.. (2015)
Endogenous Sterol Metabolites Regulate Growth of EGFR/KRAS-Dependent Tumors via LXR. Cell Rep, 12 (11): 1927-38. [PMID:26344763] |
|
5. Kim DG, Cho S, Lee KY, Cheon SH, Yoon HJ, Lee JY, Kim D, Shin KS, Koh CH, Koo JS et al.. (2021)
Crystal structures of human NSDHL and development of its novel inhibitor with the potential to suppress EGFR activity. Cell Mol Life Sci, 78 (1): 207-225. [PMID:32140747] |