GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Synthetic organic
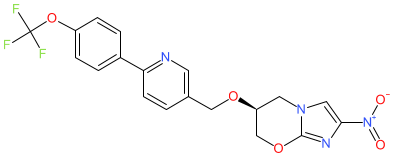
Comment: TBA-354 is a nitroimidazole-based antibacterial compound derived from pretomanid (PA-824) [1]. It has narrow-spectrum activity, inhibiting Mycobacterium tuberculosis and Mycobacterium bovis but not nontuberculous mycobacteria (NTM) or the Gram-positive and Gram-negative bacteria tested [2]. TBA-354 was under clinical development for the treatment of tuberculosis (TB), by the non-profit organisation TB Alliance (www.tballiance.org.za), but was discontinued in 2016 [3].
|
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Kmentova I, Sutherland HS, Palmer BD, Blaser A, Franzblau SG, Wan B, Wang Y, Ma Z, Denny WA, Thompson AM. (2010)
Synthesis and structure-activity relationships of aza- and diazabiphenyl analogues of the antitubercular drug (6S)-2-nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine (PA-824). J Med Chem, 53 (23): 8421-39. [PMID:21069962] |
|
2. Upton AM, Cho S, Yang TJ, Kim Y, Wang Y, Lu Y, Wang B, Xu J, Mdluli K, Ma Z et al.. (2015)
In vitro and in vivo activities of the nitroimidazole TBA-354 against Mycobacterium tuberculosis. Antimicrob Agents Chemother, 59 (1): 136-44. [PMID:25331696] |
|
3.
Phase 1 Clinical Trial of TB Drug Candidate TBA-354 Discontinued.
Accessed on 29/09/2024. Modified on 29/09/2024. TB Alliance, https://www.tballiance.org/news/phase-1-clinical-trial-tb-drug-candidate-tba-354-discontinued |