GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
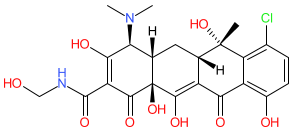
Synonyms: chlormethylenecycline | Megaclor®
clomocycline is an approved drug
Compound class:
Synthetic organic
Comment: Clomocycline is a derivative of chlortetracycline, belonging to the tetracycline class of antibacterial compounds. Note that the structure shown here matches the CAS-assigned structure. The PubChem link, provided in the table below, is to their standardized chemical structure (CID 54680675), although this is not an exact structural match.
|
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Andrew GS, Edwards JC, Oller LZ. (1969)
Clinical trial of clomocycline (Megaclor) in gonococcal and non-gonococcal urethritis. Br J Vener Dis, 45 (2): 154-6. [PMID:4892122] |
|
2. Chopra I, Hawkey PM, Hinton M. (1992)
Tetracyclines, molecular and clinical aspects. J Antimicrob Chemother, 29 (3): 245-77. [PMID:1592696] |
|
3. Chopra I, Roberts M. (2001)
Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev, 65 (2): 232-60 ; second page, table of contents. [PMID:11381101] |