GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
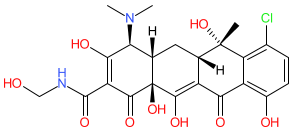
Synonyms: chlormethylenecycline | Megaclor®
clomocycline is an approved drug
Compound class:
Synthetic organic
Comment: Clomocycline is a derivative of chlortetracycline, belonging to the tetracycline class of antibacterial compounds. Note that the structure shown here matches the CAS-assigned structure. The PubChem link, provided in the table below, is to their standardized chemical structure (CID 54680675), although this is not an exact structural match.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Clomocycline was marketed in a number of countries, including Italy and the UK (trade name Megaclor®) and used to treat various bacterial infections [1]. There is no information regarding current approval for clinical use of this drug on the US FDA, European Medicines Agency (EMA) or UK (eMC) websites. Other national approval agencies may continue granting marketing authorisation. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Tetracyclines inhibit bacterial protein synthesis by binding reversibly to the bacterial 30S ribosomal subunit and preventing the aminoacyl tRNA from binding to the acceptor (A) site on the mRNA-ribosome complex (reviewed in [2-3]). |