|
Comment: MX-2401 is a lipopeptide antibacterial compound and a semisynthetic analogue of the natural product amphomycin [ 2].
|
|
2D Structure
|
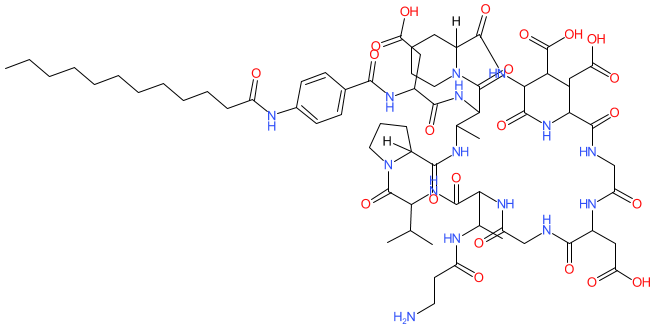
|
Physico-chemical Properties
|
|
|
Hydrogen bond acceptors
|
37
|
|
Hydrogen bond donors
|
17
|
|
Rotatable bonds
|
32
|
|
Topological polar surface area
|
565.04
|
|
Molecular weight
|
1468.61
|
|
XLogP
|
2.51
|
|
No. Lipinski's rules broken
|
4
|
Generated using the Chemistry Development Kit (CDK) (Willighagen EL et al. Journal of Cheminformatics vol. 9:33. 2017, doi:10.1186/s13321-017-0220-4; https://cdk.github.io/)
|
SMILES / InChI / InChIKey
|
|
|
Canonical SMILES
|
CCCCCCCCCCCC(=O)NC1=CC=C(C=C1)C(=O)NC(CC(=O)O)C(=O)NC2C(C)NC(=O)C3([H])CCCN3C(=O)C(C(C)C)NC(=O)C(C(C)NC(=O)CCN)NC(=O)CNC(=O)C(CC(=O)O)NC(=O)CNC(=O)C(CC(=O)O)NC(=O)C(C(C)C(=O)O)NC(=O)C4([H])CCCCN4C2=O
|
|
Isomeric SMILES
|
O=C1N2C(C(=O)NC(C(C(O)=O)C)C(=O)NC(CC(O)=O)C(=O)NCC(=O)NC(CC(O)=O)C(=O)NCC(=O)NC(C(NC(CCN)=O)C)C(=O)NC(C(C)C)C(=O)N3C(C(=O)NC(C)C1NC(C(NC(=O)C4=CC=C(NC(CCCCCCCCCCC)=O)C=C4)CC(O)=O)=O)(CCC3)[H])(CCCC2)[H]
|
|
InChI
|
InChI=1S/C67H101N15O22/c1-7-8-9-10-11-12-13-14-15-21-46(83)73-40-24-22-39(23-25-40)57(93)75-43(32-52(91)92)60(96)80-56-38(6)72-61(97)45-20-18-29-82(45)65(101)53(35(2)3)78-64(100)55(37(5)71-47(84)26-27-68)77-49(86)34-70-58(94)41(30-50(87)88)74-48(85)33-69-59(95)42(31-51(89)90)76-63(99)54(36(4)67(103)104)79-62(98)44-19-16-17-28-81(44)66(56)102/h22-25,35-38,41-45,53-56H,7-21,26-34,68H2,1-6H3,(H,69,95)(H,70,94)(H,71,84)(H,72,97)(H,73,83)(H,74,85)(H,75,93)(H,76,99)(H,77,86)(H,78,100)(H,79,98)(H,80,96)(H,87,88)(H,89,90)(H,91,92)(H,103,104)
|
|
InChI Key
|
FVWLJHLXFKFMJA-UHFFFAOYSA-N
|
Generated using the Chemistry Development Kit (CDK) (Willighagen EL et al. Journal of Cheminformatics vol. 9:33. 2017, doi:10.1186/s13321-017-0220-4; https://cdk.github.io/)
|
|







