GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: compound 28 [PMID: 38300987] | NX-2127 | NX2127
Compound class:
Synthetic organic
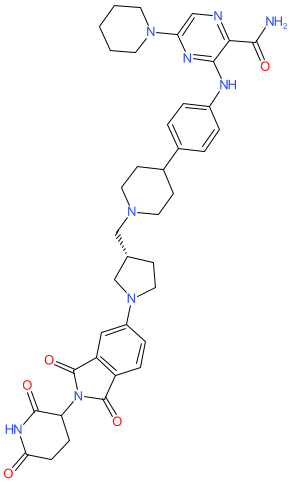
Comment: Zelebrudomide (NX-2127) is an orally bioactive PROTAC degrader of Bruton's tyrosine kinase (BTK) [1]. It contains a BTK-binding moiety linked to a thalidomide-based cereblon (CRBN) engaging moiety. Zelebrudomide acts as a dual PROTAC (through recruitment of cereblon) and molecular glue (degradation of the neosubstrate transcription factors IKZF1/Ikaros and IKZF3/Aiolos). This combined action targets BTK on B cells, and boosts T cell activity, and is predicted to offer an improved mechanism to treat B cell malignancies, even in the presence of BTK mutations that confer resistance to clinically used kinase inhibitor drugs.
The INN record notes that one of the bonds can exist as R or S epimers, so we depict this bond without specified stereo to represent the racemate. The other rotational bond is S. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| The DC50 of NX-2127 is 4.5 nM in wild type TMD8 cells, and 31 nM in TMD8 cells with the BTKC481S covalent BTK inhibitor resistance mutation [1] . |
| Selectivity at enzymes | ||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||