GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: compound of formula A [US20220024908A1] | TGRX-326 | TGRX326
Compound class:
Synthetic organic
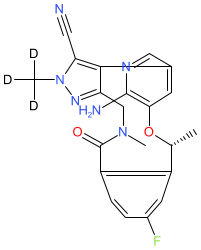
Comment: The chemical structure for deulorlatinib was obtained from WHO proposed INN list 131 (August 2024). Chemically it is a deuterated analogue of the approved dual ALK/ROS1 inhibitor lorlatinib. Deulorlatinib is claimed in Shenzen Targetrx patent US20220024908A1 [1], and we predict that deulorlatinib is the INN for their lead compound TGRX-326 (based on their disclosed pipeline and a meeting abstract [2]).
|
|
|||||||||||||||||||||||||||||||||||
Download 2D Structure  |
|
| Canonical SMILES | Download |
| Isomeric SMILES | Download |
| InChI standard identifier | Download |
| InChI standard key | Download |
Molecular structure representations generated using Open Babel