GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
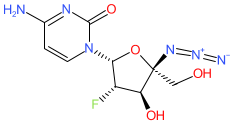
Synonyms: RO-0622
Compound class:
Synthetic organic
Comment: Azvudine is a nucleoside analogue that was first identified as an inhibitor of the HIV-1 RNA-dependent RNA polymerase (RdRp) [4]. It has more recently been reported to inhibit RdRp from the SARS-CoV-2 betacoronavirus [5]. The active triphosphate metabolite concentrates in the thymus and peripheral blood mononuclear cells, and oral treatment induces curative mechanisms in preclinical models.
|
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| Azvudine inhibits SARS-CoV-2 and HCoV-OC43 replication in vitro (EC50 1.2-4.3 μM) [5]. |
| Selectivity at other protein targets | ||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||