GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Synthetic organic
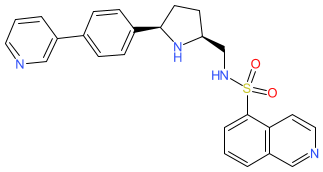
Comment: LEI-800 is the optimized lead from a novel class of isoquinoline sulfonamide antibacterial compounds [2]. The compound inhibits bacterial DNA gyrase and has activity against clinically relevant Gram-negative bacteria.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Mechanism Of Action and Pharmacodynamic Effects  |
| LEI-800 is a potent inhibitor of DNA gyrase, a type II DNA topoisomerase that catalyzes the negative supercoiling of DNA and is essential in all bacteria but absent from higher eukaryotes [1,3]. Analysis of cryo-EM structures reveals that LEI-800 binds to both DNA gyrase A subunits, within a hydrophobic pocket on the DNA-binding surface, a mode of action that is distinct from that of previously described DNA gyrase inhibitors [2]. |