GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
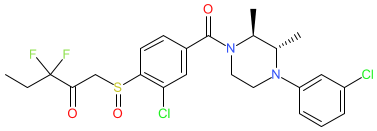
Synonyms: compound ±73 [Jiang et al., 2024] | LEI515
Compound class:
Synthetic organic
Comment: LEI-515 is a reversible monoacylglycerol lipase (MAGL) inhibitor [1]. Its pharmacodynamics restrict its inhibitory effect to peripheral MAGL, thus reducing the risk of side effects mediated by inhibition of MAGL in the CNS. LEI-515 reduces neuropathic pain and inflammation in preclinical models [2].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Jiang M, Huizenga MCW, Mohr F, Amedi A, Bakker R, van den Berg RJBHN, Deng H, van der Wel T, van Boeckel CAA, van der Stelt M. (2024)
Structure–Activity Relationship Studies of Aryl Sulfoxides as Reversible Monoacylglycerol Lipase Inhibitors. J Med Chem,. DOI: 10.1021/acs.jmedchem.4c01037 |
|
2. Jiang M, Huizenga MCW, Wirt JL, Paloczi J, Amedi A, van den Berg RJBHN, Benz J, Collin L, Deng H, Di X et al.. (2023)
A monoacylglycerol lipase inhibitor showing therapeutic efficacy in mice without central side effects or dependence. Nat Commun, 14 (1): 8039. [PMID:38052772] |