GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Synthetic organic
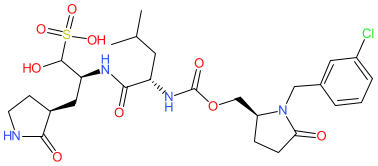
Comment: This compound was designed as a direct-acting antiviral that targets Coronavirus 3CL proteases (main protease, or Mpro) [1]. In an enzyme activity assay it exhibits ~5-fold selectivity for MERS-CoV Mpro compared to the SARS-CoV-2 isozyme. It was synthesised as the sodium sulfonate, but we show the sulfonic acid parent molecule.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Dampalla CS, Kim Y, Zabiegala A, Howard DJ, Nguyen HN, Madden TK, Thurman HA, Cooper A, Liu L, Battaile KP et al.. (2024)
Structure-Guided Design of Potent Coronavirus Inhibitors with a 2-Pyrrolidone Scaffold: Biochemical, Crystallographic, and Virological Studies. J Med Chem, 67 (14): 11937-11956. [PMID:38953866] |