GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
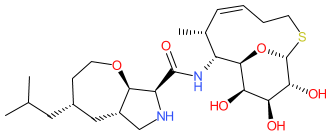
Compound class:
Synthetic organic
|
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| Cresomycin has potent in vitro activity against both Gram-positive and Gram-negative bacteria, including Staphylococcus aureus (MIC in the range of ≤0.06-1 μg/ml) and Escherichia coli (MIC in the range of 0.5-1 μg/ml) [2]. It also demonstrates in vivo efficacy with 100% survival, following subcutaneous treatment with cresomycin, of mice with systemic infections of cfr-expressing S. aureus [2]. |