GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
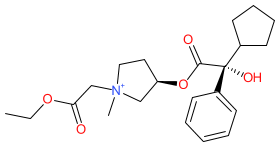
Synonyms: BBI-4000 (sofpironium bromide) | Ecclock® | Sofdra®
sofpironium is an approved drug (Japan (2020))
Compound class:
Synthetic organic
Comment: Sofpironium is an anticholinergic drug. It is a structural analogue of glycopyrrolate which was modified to be rapidly converted to an inactive metabolite in the circulation so as to restrict action to local sites of administration (i.e. it is a so-called retrometabolic or "soft" drug) and minimise potential systemic side-effects. In relation to its use to reduce sweating in axillary hyperhidrosis its effect is mediated by antagonism of M3 muscarinic acetylcholine receptors in eccrine glands.
|
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Fujimoto T, Abe Y, Igarashi M, Ishikoh A, Omi T, Kanda H, Kitahara H, Kinoshita M, Nakasu I, Hattori N et al.. (2021)
A phase III, 52-week, open-label study to evaluate the safety and efficacy of 5% sofpironium bromide (BBI-4000) gel in Japanese patients with primary axillary hyperhidrosis. J Dermatol, 48 (8): 1149-1161. [PMID:34041788] |
|
2. Paik J. (2020)
Sofpironium Bromide: First Approval. Drugs, 80 (18): 1981-1986. [PMID:33236266] |
|
3. Yokozeki H, Fujimoto T, Abe Y, Igarashi M, Ishikoh A, Omi T, Kanda H, Kitahara H, Kinoshita M, Nakasu I et al.. (2021)
A phase 3, multicenter, randomized, double-blind, vehicle-controlled, parallel-group study of 5% sofpironium bromide (BBI-4000) gel in Japanese patients with primary axillary hyperhidrosis. J Dermatol, 48 (3): 279-288. [PMID:33410265] |