GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
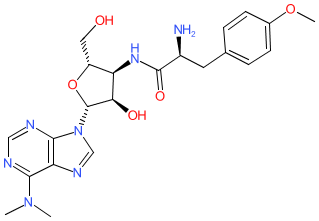
Synonyms: achromycin
Compound class:
Natural product
Comment: Puromycin is an aminoacyl nucleoside antimicrobial compound, originally isolated from Streptomyces alboniger [4]. It is a potent inhibitor of protein translation in both eukaryotic and prokaryotic organisms [5] and has not been of clinical utility due to its non-selective systemic toxicity. Instead it is used extensively in the laboratory to study mechanisms of protein synthesis and also as a selective agent in mammalian cell culture systems (reviewed in [2]).
Please note that puromycin was formerly known as achromycin, which was also a trade name used for tetracycline. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Aeluri R, Ganji RJ, Marapaka AK, Pillalamarri V, Alla M, Addlagatta A. (2015)
Highly functionalized tetrahydropyridines are cytotoxic and selective inhibitors of human puromycin sensitive aminopeptidase. Eur J Med Chem, 106: 26-33. [PMID:26513642] |
|
2. Aviner R. (2020)
The science of puromycin: From studies of ribosome function to applications in biotechnology. Comput Struct Biotechnol J, 18: 1074-1083. [PMID:32435426] |
|
3. Vara JA, Portela A, Ortín J, Jiménez A. (1986)
Expression in mammalian cells of a gene from Streptomyces alboniger conferring puromycin resistance. Nucleic Acids Res, 14 (11): 4617-24. [PMID:3714487] |
|
4. Waller CW, Fryth PW, Hutchings BL, Williams JH. (1953)
Achromycin. The structure of the antibiotic puromycin. I. J. Am. Chem. Soc., 75 (8): 2025. DOI: 10.1021/ja01104a537 |
|
5. Yarmolinsky MB, Haba GL. (1959)
INHIBITION BY PUROMYCIN OF AMINO ACID INCORPORATION INTO PROTEIN. Proc Natl Acad Sci U S A, 45 (12): 1721-9. [PMID:16590564] |