GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
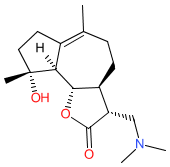
Synonyms: ACT-001
Compound class:
Synthetic organic
Comment: ACT001 inhibits PD-L1 expression in neutrophils, and releases immune system suppression by the PD-1/PD-L1 checkpoint. It has potent anticancer and anti-inflammatory activities, and can cross the blood-brain barrier. The molecular mechanism of ACT001 is reported to involve direct interaction with the STAT3 transcription factor and inhibition of STAT3 phosphorylation [3,5]. In cancers that overexpress PD-L1 and escape immune surveillance, this offers a therapeutic strategy to enhance anti-tumour immune response. Some microbial pathogens upregulate PD-L1 expression in their hosts as a mechanism to negatively regulate the host anti-pathogen immune response [1-2,4,6-7]. By blocking PD-L1 expression ACT001 has been shown effective against systemic C. albicans infection [7]. However ACT001's high CNS permeability and strong cytotoxicity limit its use in the treatment of microbial infections. A sulphide ACT001 prodrug with an improved safety profile has been designed [3].
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT05053880 | A Study to Evaluate Safety and Efficacy of ACT001 and Anti-PD-1 in Patients With Surgically Accessible Recurrent Glioblastoma Multiforme | Phase 1/Phase 2 Interventional | Accendatech USA Inc. | ||