GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Synthetic organic
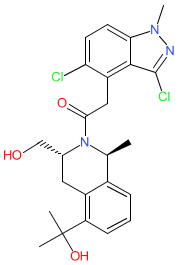
Comment: The chemical structure for glovadalen was obtained from proposed INN list 130 (Feb. 2024), in which the compound is described as a dopamine D1 receptor positive allosteric modulator (PAM). It is claimed in UCB Biopharma's patent WO2021001288A1 [1]. Exploration of UCB's declared pipeline suggests that glovadalen may be their clinical lead UCB0022, that is being developed for Parkinson's disease [2]. We will update this entry when the structure for UCB0022 and/or glovadalen is published.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| UCB0022 is a clinical candidate for the treatment of motor fluctuations and dyskinesia in Parkinson's disease, as an alternative to dopamine-replacement therapies. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT06055985 | A Study to Evaluate the Efficacy, Safety, Tolerability, and Pharmacokinetics of UCB0022 in Study Participants With Advanced Parkinson's Disease | Phase 2 Interventional | UCB Pharma | ||
| NCT04867642 | A Study to Test the Safety, Tolerability, and Blood Levels of UCB0022 in Healthy Participants and Participants With Parkinson's Disease | Phase 1 Interventional | UCB Pharma | ||