GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
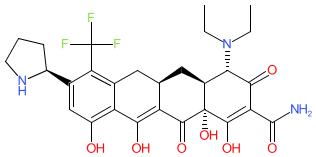
Compound class:
Synthetic organic
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| TP-6076 completed a Phase 1 trial to evaluate safety and pharmacokinetics in healthy participants (NCT03691584). However, development may have ceased following acquisition of Tetraphase Pharmaceuticals by La Jolla Pharmaceutical Company, who are now part of Innoviva. TP-6076 is not included on Innoviva's pipeline webpage. |
Mechanism Of Action and Pharmacodynamic Effects  |
| TP-6076 inhibits bacterial protein synthesis by binding to the bacterial 30S ribosomal subunit and blocking binding of aminoacyl tRNA to mRNA. The structure of TP-6076, bound to the A. baumannii ribosome and the Acinetobacter drug efflux pump AdeJ has been elucidated [2]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03691584 | Phase 1, Safety and Bronchopulmonary PK Study in Healthy Volunteers | Phase 1 Interventional | La Jolla Pharmaceutical Company | ||