GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Synthetic organic
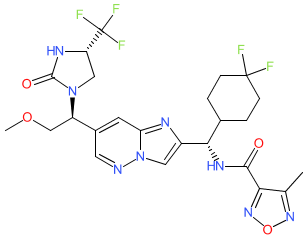
Comment: LY3509754 is an orally bioavailable, small molecule inhibitor of the proinflammatory cytokine IL-17A [1]. It was intended as an alternative to anti-IL-17A monoclonals for the treatment of psoriasis.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| A first-in-humans safety, tolerability and pharmacokinetics trial was terminated due to safety concerns (drug-induced liver injury) [1]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04586920 | A Study of LY3509754 in Healthy Non-Japanese and Japanese Participants | Phase 1 Interventional | Eli Lilly and Company | Despite strong target engagement orally administered LY3509754 was not well tolerated in human volunteers [1]. | |