GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: 14,539 RP | Solosec® | SYM-1219
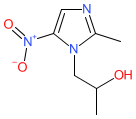
secnidazole is an approved drug (FDA (2017))
Compound class:
Synthetic organic
Comment: Secnidazole is a broad-spectrum nitroimidazole antimicrobial compound that exhibits antiprotozoal and antibacterial (Gram-positive and Gram-negative) activities.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| In September 2017, the US FDA granted secnidazole oral granules approval for the treatment of bacterial vaginosis. In June 2021, the FDA expanded approval to include treatment of trichomoniasis caused by Trichomonas vaginalis. It is also available in other countries under various trade names, click here to link to Drugs.com's list of internationally marketed secnidazole drugs. |
Pharmacokinetics  |
| Elimination |
| Secnidazole has a longer serum half-life (mean of 17.0 h) than other prescribed nitroimidazoles [3]. |
External links  |
|
For extended ADME data see the following: Drugs.com |