GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
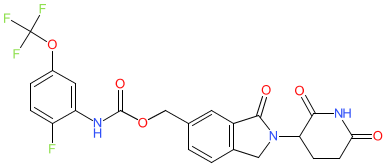
Synonyms: MRT2359
Compound class:
Synthetic organic
Comment: MRT-2359 is a GSPT1 (a.k.a. eRF3a)-targeting molecular glue degrader. It uses a cereblon (CRBN) binding moiety to induce degradation of the translation termination factor GSPT1 (GSPT1; P15170). The degrader was developed to target MYC-driven tumours that are thought to become dependent on GSPT1. MRT-2359 induces an anti-proliferative effect on cancer cells.
The chemical structure for MRT-2359 was disclosed during the 'First Disclosures of New Drug Candidates' session at the April 2023 (Orlando) meeting of the AACR. |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT05546268 | Study of Oral MRT-2359 in Selected Cancer Patients | Phase 1/Phase 2 Interventional | Monte Rosa Therapeutics, Inc | ||