GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: Stachybotrys microspora triprenyl phenol 7 | BIIB131 | SMTP7 | TMS-007
Compound class:
Natural product
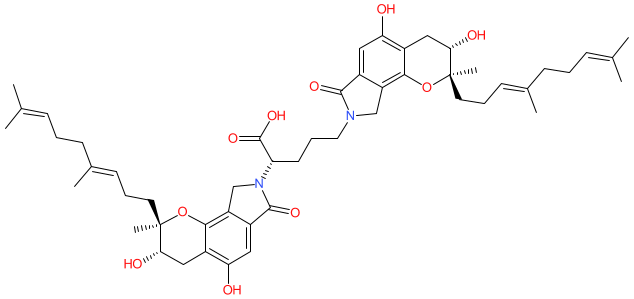
Comment: SMTP-7 is a natural triprenyl phenol that's produced by Stachybotrys microspora (a fungus) [2]. It is a complex molecule with 5 chiral centers whose absolute stereochemical configuration has been determined using microcrystal electron diffraction (MicroED) [7]. Total chemical synthesis has been reported [3]. SMTP-7 produces thrombolytic [5], anti-inflammatory, and antioxidant effects. It is being considered as a clinical anti-thrombotic agent for use in stroke patients [6]. The compound has activity as a plasminogen modulator and soluble epoxide hydrolase inhibitor that may offer a novel therapeutic intervention for cardiometabolic diseases [1].
|
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Bellien J, Djerada Z. (2022)
Use of inhibitors of phosphatase activity of soluble epoxide for the treatment of cardiometabolic diseases. Patent number: US20220023265A1. Assignee: Universitaire De Reims. Priority date: 16/09/2019. Publication date: 27/01/2022. |
|
2. Hasumi K, Yamamichi S, Harada T. (2010)
Small-molecule modulators of zymogen activation in the fibrinolytic and coagulation systems. FEBS J, 277 (18): 3675-87. [PMID:20718867] |
|
3. Kwok DA, Huang R, Shang D, Xue Y, Walker DG. (2024)
Total Synthesis of Stachybotrys microspora Triprenol Phenol-7 (SMTP-7). Org Lett, 26 (3): 697-701. [PMID:38232331] |
|
4. Moritoyo T, Nishimura N, Hasegawa K, Ishii S, Kirihara K, Takata M, Svensson AK, Umeda-Kameyama Y, Kawarasaki S, Ihara R et al.. (2023)
A first-in-human study of the anti-inflammatory profibrinolytic TMS-007, an SMTP family triprenyl phenol. Br J Clin Pharmacol, 89 (6): 1809-1819. [PMID:36562925] |
|
5. Sawada H, Nishimura N, Suzuki E, Zhuang J, Hasegawa K, Takamatsu H, Honda K, Hasumi K. (2014)
SMTP-7, a novel small-molecule thrombolytic for ischemic stroke: a study in rodents and primates. J Cereb Blood Flow Metab, 34 (2): 235-41. [PMID:24192639] |
|
6. Suzuki E, Nishimura N, Yoshikawa T, Kunikiyo Y, Hasegawa K, Hasumi K. (2018)
Efficacy of SMTP-7, a small-molecule anti-inflammatory thrombolytic, in embolic stroke in monkeys. Pharmacol Res Perspect, 6 (6): e00448. [PMID:30546909] |
|
7. Wang B, Lin Y. (2022)
Absolute configuration determination of SMTP-7 via microcrystal electron diffraction (MicroED). Chem Commun (Camb), 58 (94): 13071-13074. [PMID:36305866] |