GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
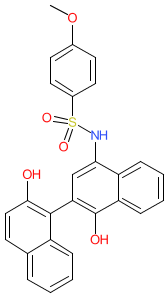
Synonyms: C188-9 [2] | TTI 101 | TTI101
Compound class:
Synthetic organic
Comment: TTI-101 (Tvardi Therapeutics; formerly C188-9) is an orally bioavailable STAT3 transcription factor inhibitor. It blocks the tyrosine residue phosphorylation by Janus kinase (JAK) that mediates STAT3 activation, leading to downregulation of STAT3-mediated gene expression. This mechanism of action is considered as a valid approach for the treatment of inflammation, fibrosis, and a range of cancers [1,3]. The follow-on compound TTI-109, that is chemically distinct from TTI-101, has completed IND-enabling studies.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| TTI-101 was advanced as a clinical lead for cancers and fibrotic diseases. In October 2025, Tvardi announced poor preliminary data from their phase 2 idiopathic pulmonary fibrosis (IPF) study, which suffered from high participant dropout rates (>55%, principally due to gastrointestinal adverse events) and placebo-like efficacy [5]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT05440708 | A Study of TTI-101 as Monotherapy and in Combination in Participants With Locally Advanced or Metastatic, and Unresectable Hepatocellular Carcinoma | Phase 1/Phase 2 Interventional | Tvardi Therapeutics, Incorporated | ||
| NCT05384119 | Study of TTI-101 in Combination for Participants With Metastatic Hormone Receptor-Positive and Human Epithelial Receptor 2 (HER2)-Negative Breast Cancer | Phase 1/Phase 2 Interventional | Tvardi Therapeutics, Incorporated | ||
| NCT03195699 | Oral STAT3 Inhibitor, TTI-101, in Patients With Advanced Cancers | Phase 1 Interventional | Tvardi Therapeutics, Incorporated | 4 | |
| NCT05671835 | Study of TTI-101 in Participants With Idiopathic Pulmonary Fibrosis | Phase 2 Interventional | Tvardi Therapeutics, Incorporated | The REVERT-IPF study | |