|
Synonyms: aminosidine | catenulin | Humatin® | monomycin A
paromomycin is an approved drug (FDA (1969))
Compound class:
Natural product
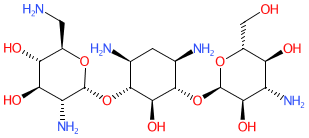
Comment: Paromomycin belongs to the aminoglycoside class of antibacterial compounds and was originally isolated from Streptomyces krestomuceticus. It is used as an antiprotozoal drug and is included in the World Health Organization's Model List of Essential Medicines (link provided in the Classification table, under the Summary tab below).
|
|
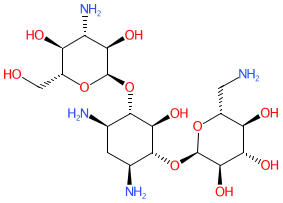
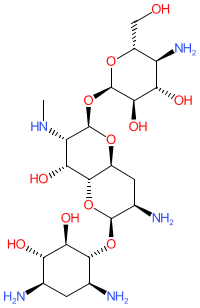
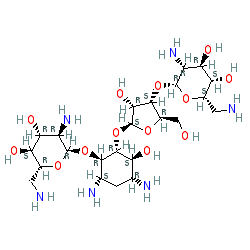
2D Structure
|
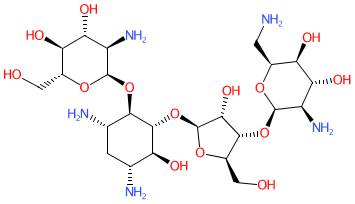
|
Physico-chemical Properties
|
|
|
Hydrogen bond acceptors
|
19
|
|
Hydrogen bond donors
|
13
|
|
Rotatable bonds
|
9
|
|
Topological polar surface area
|
347.32
|
|
Molecular weight
|
615.3
|
|
XLogP
|
-6.85
|
|
No. Lipinski's rules broken
|
2
|
Generated using the Chemistry Development Kit (CDK) (Willighagen EL et al. Journal of Cheminformatics vol. 9:33. 2017, doi:10.1186/s13321-017-0220-4; https://cdk.github.io/)
|
SMILES / InChI / InChIKey
|
|
|
Canonical SMILES
|
OC[C@H]1O[C@H]([C@@H]([C@@H]1O[C@H]1O[C@@H](CN)[C@H]([C@@H]([C@H]1N)O)O)O)O[C@@H]1[C@@H](O)[C@H](N)C[C@@H]([C@H]1O[C@H]1O[C@H](CO)[C@H]([C@@H]([C@H]1N)O)O)N
|
|
Isomeric SMILES
|
C1[C@H]([C@@H]([C@H]([C@@H]([C@H]1N)O[C@@H]1[C@@H]([C@H]([C@@H]([C@H](O1)CO)O)O)N)O[C@H]1[C@@H]([C@@H]([C@H](O1)CO)O[C@@H]1[C@@H]([C@H]([C@@H]([C@@H](O1)CN)O)O)N)O)O)N
|
|
InChI
|
InChI=1S/C23H45N5O14/c24-2-7-13(32)15(34)10(27)21(37-7)41-19-9(4-30)39-23(17(19)36)42-20-12(31)5(25)1-6(26)18(20)40-22-11(28)16(35)14(33)8(3-29)38-22/h5-23,29-36H,1-4,24-28H2/t5-,6+,7+,8-,9-,10-,11-,12+,13-,14-,15-,16-,17-,18-,19-,20-,21-,22-,23+/m1/s1
|
|
InChI Key
|
UOZODPSAJZTQNH-LSWIJEOBSA-N
|
Generated using the Chemistry Development Kit (CDK) (Willighagen EL et al. Journal of Cheminformatics vol. 9:33. 2017, doi:10.1186/s13321-017-0220-4; https://cdk.github.io/)
|
|