GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
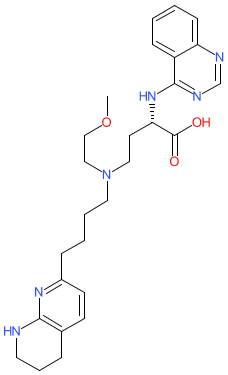
Synonyms: PLN-74809 | PLN74809
Compound class:
Synthetic organic
Comment: Bexotegrast (PLN-74809; Pliant Therapeutics) is a dual-selective integrin inhibitor/antagonist that was developed for anti-fibrosis potential [1]. The αv integrins regulate TGFβ activation (catalysing conversion of latent TGFβ isoforms to the active forms) and fibrogenesis in pulmonary fibrosis models [2-3], and αvβ6 and αvβ1 integrins are expressed on abnormal epithelial cells and fibroblasts (respectively) in human fibrotic lung tissue. By antagonising the function of both of these, bexotegrast restricts TGFβ activation and downstream signalling, which has been shown to promote an anti-fibrogenic effect in lung tissue samples from patients with idiopathic pulmonary fibrosis (IPF) [1].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Bexotegrast (PLN-74809) has been advanced to phase 2 studies in participants with chronic fibrotic lung or liver diseases. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04072315 | Phase 2a Evaluation of PLN-74809 on αvβ6 Receptor Occupancy Using PET Imaging in Participants With IPF/ | Phase 2 Interventional | Pliant Therapeutics, Inc. | ||
| NCT04396756 | Evaluation of Efficacy and Safety of PLN-74809 in Patients With Idiopathic Pulmonary Fibrosis | Phase 2 Interventional | Pliant Therapeutics, Inc. | ||
| NCT04480840 | Phase 2a Evaluation of Safety, Tolerability, and Pharmacokinetics of PLN-74809 in Patients With Primary Sclerosing Cholangitis (PSC) | Phase 2 Interventional | Pliant Therapeutics, Inc. | ||
| NCT04565249 | Evaluation of the Safety, Tolerability, and Pharmacokinetics of PLN-74809 in Participants With Acute Respiratory Distress Syndrome (ARDS) Associated With at Least Severe COVID-19 | Phase 2 Interventional | Pliant Therapeutics, Inc. | This trial was terminated with only 6 volunteers recruited. Participants are no longer receiving treatment or being monitored. | |