GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: FK-037 | Wincef®
cefoselis is an approved drug (Japan)
Compound class:
Synthetic organic
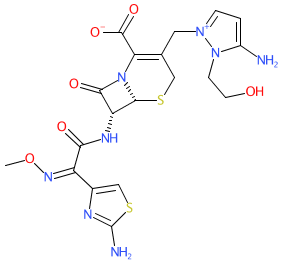
Comment: Cefoselis is a semisynthetic, fourth generation cephalosporin belonging to the β-lactam class of antibacterial compounds.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| The first marketing authorisation for cefoselis was issued in Japan in 1998. |