GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
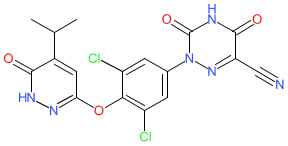
Synonyms: compound 53 [PMID: 24712661] | MGL-3196 | MGL3196 | Rezdiffra® | VIA-3196 | VIA3196
resmetirom is an approved drug (FDA (2024))
Compound class:
Synthetic organic
Comment: Resmetirom (MGL-3196) was developed as a thyroid receptor β (THRβ)-selective agonist, for potential to treat non-alcoholic steatohepatitis (NASH) and heterozygous familial hypercholesterolemia, with reduced potential to cause side-effects associated with THRα activation outside the liver [7].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Harrison SA, Bashir M, Moussa SE, McCarty K, Pablo Frias J, Taub R, Alkhouri N. (2021)
Effects of Resmetirom on Noninvasive Endpoints in a 36-Week Phase 2 Active Treatment Extension Study in Patients With NASH. Hepatol Commun, 5 (4): 573-588. [PMID:33860116] |
|
2. Harrison SA, Bashir MR, Guy CD, Zhou R, Moylan CA, Frias JP, Alkhouri N, Bansal MB, Baum S, Neuschwander-Tetri BA et al.. (2019)
Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet, 394 (10213): 2012-2024. [PMID:31727409] |
|
3. Harrison SA, Bedossa P, Guy CD, Schattenberg JM, Loomba R, Taub R, Labriola D, Moussa SE, Neff GW, Rinella ME et al.. (2024)
A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N Engl J Med, 390 (6): 497-509. [PMID:38324483] |
|
4. Harrison SA, Ratziu V, Anstee QM, Noureddin M, Sanyal AJ, Schattenberg JM, Bedossa P, Bashir MR, Schneider D, Taub R et al.. (2024)
Design of the phase 3 MAESTRO clinical program to evaluate resmetirom for the treatment of nonalcoholic steatohepatitis. Aliment Pharmacol Ther, 59 (1): 51-63. [PMID:37786277] |
|
5. Harrison SA, Taub R, Neff GW, Lucas KJ, Labriola D, Moussa SE, Alkhouri N, Bashir MR. (2023)
Resmetirom for nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled phase 3 trial. Nat Med, 29 (11): 2919-2928. [PMID:37845512] |
|
6. Keam SJ. (2024)
Resmetirom: First Approval. Drugs, 84 (6): 729-735. [PMID:38771485] |
|
7. Kelly MJ, Pietranico-Cole S, Larigan JD, Haynes NE, Reynolds CH, Scott N, Vermeulen J, Dvorozniak M, Conde-Knape K, Huang KS et al.. (2014)
Discovery of 2-[3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl]-3,5-dioxo-2,3,4,5-tetrahydro[1,2,4]triazine-6-carbonitrile (MGL-3196), a Highly Selective Thyroid Hormone Receptor β agonist in clinical trials for the treatment of dyslipidemia. J Med Chem, 57 (10): 3912-23. [PMID:24712661] |
|
8. Ray K. (2024)
Resmetirom proves positive for NASH with liver fibrosis. Nat Rev Gastroenterol Hepatol, 21 (4): 218. [PMID:38360990] |
|
9. Ray K. (2024)
Resmetirom safe for nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol, 21 (1): 2. [PMID:37990099] |
|
10. Younossi ZM, Stepanova M, Taub RA, Barbone JM, Harrison SA. (2022)
Hepatic Fat Reduction Due to Resmetirom in Patients With Nonalcoholic Steatohepatitis Is Associated With Improvement of Quality of Life. Clin Gastroenterol Hepatol, 20 (6): 1354-1361.e7. [PMID:34329774] |