GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: GDC-6036 | GDC6036 | RG-6330 | RG6330
Compound class:
Synthetic organic
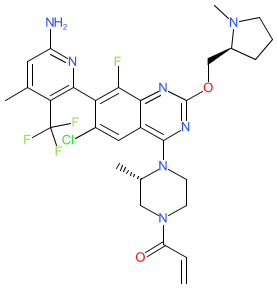
Comment: GDC-6036 is an oral, covalent inhibitor that targets oncogenic KRASG12C [2-3]. Inhibitor binding locks KRASG12C into its inactive GDP-bound state. GDC-6036's chemical structure was revealed at the AACR spring meeting in 2022. The structure is claimed i patent US11236068B2 [1], and [3] provides the chemical name 1-((S)-4-((R)-7-(6-Amino-4-methyl-3-(trifluoromethyl)pyridin-2-yl)-6-chloro-8-fluoro-2-(((S)-1- methylpyrrolidin-2-yl)methoxy)quinazolin-4-yl)-3-methylpiperazin-1-yl)prop-2-en-1-one (adipiate salt). We matched GDC-6036's chemical structure to the INN 'divarasib' which was published in the WHO's proposed INN list 127 on 21 July 2022.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| GDC-6036 will be evaluated for efficacy in advanced KRASG12C positive solid tumours. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04449874 | A Study to Evaluate the Safety, Pharmacokinetics, and Activity of GDC-6036 Alone or in Combination in Participants With Advanced or Metastatic Solid Tumors With a KRAS G12C Mutation | Phase 1 Interventional | Genentech, Inc. | ||
| NCT03178552 | A Study to Evaluate the Efficacy and Safety of Multiple Targeted Therapies as Treatments for Participants With Non-Small Cell Lung Cancer (NSCLC) | Phase 2/Phase 3 Interventional | Hoffmann-La Roche | ||