GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms:
Compound class:
Synthetic organic
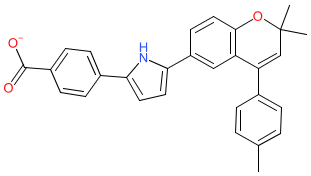
Comment: YCT-529 is an oral retinoic acid receptor alpha (RAR-α; RARA) antagonist [4]. Its development and function were originally presented at the spring meeting of the American Chemical Society (ACS) in March 2022. YCT-529 has no detectable agonist activity, unlike a previously reported compound BMS-189453 which is an antagonist/partial agonist [3]. Antagonising RAR-α disrupts spermatogenesis.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| YCT-529 has progressed to clinical evaluation to determine safety and efficacy as an orally administered male contraceptive. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT06542237 | Open Label, Repeat Dose Study Evaluating YCT-529 in Healthy Males | Phase 1/Phase 2 Interventional | YourChoice Therapeutics, Inc. | ||
| NCT06094283 | First in Human Study Evaluating Single Ascending Oral Doses of YCT-529 in Healthy Males | Phase 1 Interventional | YourChoice Therapeutics, Inc. | 2 | |