GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
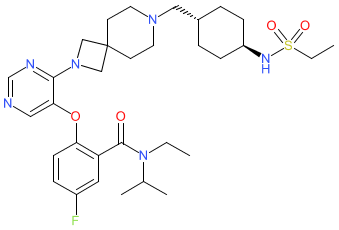
Synonyms: Revuforj® | SNDX-5613 | SNDX5613
revumenib is an approved drug (FDA (2024))
Compound class:
Synthetic organic
Comment: The chemical structure for revumenib was revealed in WHO proposed INN list 126 (Jan 2022), in which it was described as an antineoplastic agent. It resolved to Syndax Pharmaceuticals' SNDX-5613 via a PubChem match on SMILES. SNDX-5613 inhbits the Menin-KMT2A protein-protein interaction (binding directly to menin), as a strategy that was originally proposed to treat acute leukemias that are driven by proleukemogenic KMT2A rearrangements [1].
Additional menin inhibitors in development include bleximenib (JNJ-75276617), ziftomenib (KO-539), emilumenib (DS-1594a), enzomenib (DSP-5336) and icovamenib (BMF-219). |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Fiskus W, Boettcher S, Daver N, Mill CP, Sasaki K, Birdwell CE, Davis JA, Takahashi K, Kadia TM, DiNardo CD et al.. (2022)
Effective Menin inhibitor-based combinations against AML with MLL rearrangement or NPM1 mutation (NPM1c). Blood Cancer J, 12 (1): 5. [PMID:35017466] |
|
2. Issa GC, Aldoss I, Thirman MJ, DiPersio J, Arellano M, Blachly JS, Mannis GN, Perl A, Dickens DS, McMahon CM et al.. (2025)
Menin Inhibition With Revumenib for KMT2A-Rearranged Relapsed or Refractory Acute Leukemia (AUGMENT-101). J Clin Oncol, 43 (1): 75-84. [PMID:39121437] |
|
3. Syed YY. (2025)
Revumenib: First Approval. Drugs, 85 (4): 577-583. [PMID:40072775] |