GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
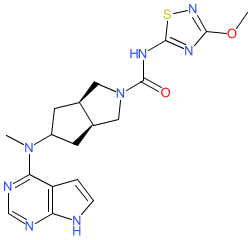
Synonyms: example 34 [WO2013091539A1] [8] | SHR-0302 | SHR0302
ivarmacitinib is an approved drug
Compound class:
Synthetic organic
Comment: Ivarmacitinib (SHR0302) is an oral Janus kinase (JAK) inhibitor, with selectivity for JAK1. It is being investigated for anti-inflammatory potential in auto-inflammatory diseases [6-7,10]. Likely to be administered as the bisulfate salt as outlined in patent WO2014194741A1 [5].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Chen B, Zhong J, Li X, Pan F, Ding Y, Zhang Y, Chen H, Liu F, Zhang Z, Zhang L et al.. (2022)
Efficacy and Safety of Ivarmacitinib in Patients With Moderate-to-Severe, Active, Ulcerative Colitis: A Phase II Study. Gastroenterology, 163 (6): 1555-1568. [PMID:35963369] |
|
2. Keam SJ. (2025)
Ivarmacitinib Sulfate: First Approval. Drugs, 85 (9): 1163-1170. [PMID:40542222] |
|
3. Liu J, Jiang Y, Zhang S, Liu S, Su J, Lin C, He X, Wu R, Yang L, Liu H et al.. (2025)
Ivarmacitinib, a selective Janus kinase 1 inhibitor, in patients with moderate-to-severe active rheumatoid arthritis and inadequate response to conventional synthetic DMARDs: results from a phase III randomised clinical trial. Ann Rheum Dis, 84 (2): 188-200. [PMID:39919893] |
|
4. Liu X, Xu L, Zhao C, Liu S, Sun L, Yang L, Xu X, Wu R, Liu X, Zhang J et al.. (2025)
Efficacy and Safety of Ivarmacitinib, a Selective JAK1 Inhibitor, in Patients With Active Ankylosing Spondylitis: Results From an Adaptive Seamless Phase 2/3 Trial. Arthritis Rheumatol, 77 (11): 1512-1523. [PMID:40509748] |
|
5. Sun P, Wu J, Gao X, Shen L. (2014)
Bisulfate of janus kinase (jak) inhibitor and preparation method therefor. Patent number: WO2014194741A1. Assignee: Jiangsu Hengrui Pharmaceutical Co., Ltd.. Priority date: 07/06/2013. Publication date: 11/12/2014. |
|
6. Sun X, He Q, Yang J, Wang A, Zhang F, Qiu H, Zhou K, Wang P, Ding X, Yuan X et al.. (2021)
Preventive and Therapeutic Effects of a Novel JAK Inhibitor SHR0302 in Acute Graft-Versus-Host Disease. Cell Transplant, 30: 9636897211033778. [PMID:34269100] |
|
7. Wu H, Yan S, Chen J, Luo X, Li P, Jia X, Dai X, Wang C, Huang Q, Liu L et al.. (2016)
JAK1-STAT3 blockade by JAK inhibitor SHR0302 attenuates inflammatory responses of adjuvant-induced arthritis rats and decreases Th17 and total B cells. Joint Bone Spine, 83 (5): 525-32. [PMID:26832189] |
|
8. Zhang X, Dong Q, Liu B, Zhu Y, Li X, Lan J. (2013)
Pyrrole six-membered heteroaryl ring derivative, preparation method therefor, and medicinal uses thereof. Patent number: WO2013091539A1. Assignee: Jiangsu Hengrui Medicine Co., Ltd., Shanghai Hengrui Medicine Co., Ltd.. Priority date: 21/12/2011. Publication date: 27/06/2013. |
|
9. Zhao Y, Gooderham M, Yang B, Wu J, Wu L, Loo WJ, Toth D, Sauder M, Li J, Chen A et al.. (2025)
Ivarmacitinib for Moderate to Severe Atopic Dermatitis in Adults and Adolescents: A Phase 3 Randomized Clinical Trial. JAMA Dermatol, 161 (7): 688-697. [PMID:40305055] |
|
10. Zhao Y, Zhang L, Ding Y, Tao X, Ji C, Dong X, Lu J, Wu L, Wang R, Lu Q et al.. (2021)
Efficacy and Safety of SHR0302, a Highly Selective Janus Kinase 1 Inhibitor, in Patients with Moderate to Severe Atopic Dermatitis: A Phase II Randomized Clinical Trial. Am J Clin Dermatol, 22 (6): 877-889. [PMID:34374027] |