GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
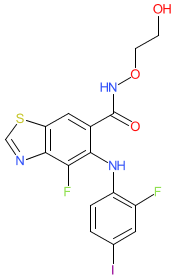
Synonyms: Example 9 [WO2013107283A1] | HL-085 | HL085 | Kolupin® (China)
tunlametinib is an approved drug (China (2024))
Compound class:
Synthetic organic
Comment: We obtained the chemical structure for tunlametinib from WHO Proposed list 125 (July 2021). This mapped to PubChem CID 71621329. The chemical structure is claimed in Tianjin Binjiang Pharma's patent WO2013107283A1 for activity as a mitogen-activated protein kinase kinase (MEK) inhibitor with potential to treat cancers and inflammation [4]. The name-to-structure was confirmed by published disclosure in September 2023 [2], and MMOA was reported as a MEK1/2 inhibitor.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Tunlametinib (HL-085) was progressed as a clinical candidate for solid tumours with RAS and RAF mutations. First (conditional) approval was granted by the Chinese NMPA in March 2024, for the treatment of NRAS-mutated advanced melanoma that is unresponsive to anti-PD-1/PD-L1 immune checkpoint therapy [1,5]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT05331105 | HL-085 in Adults With Neurofibromatosis Type 1 (NF1) and Inoperable Plexiform Neurofibromas | Phase 2 Interventional | Shanghai Kechow Pharma, Inc. | ||
| NCT03973151 | Study of HL-085 in NRAS Mutant Advanced Melanoma | Phase 1/Phase 2 Interventional | Shanghai Kechow Pharma, Inc. | ||
| NCT05217303 | HL-085 in NRAS-mutated Advanced Melanoma | Phase 2 Interventional | Shanghai Kechow Pharma, Inc. | ||
| NCT03781219 | A PhaseI Study of HL-085 Plus Vemurafenib in Solid Tumor With BRAF V600 Mutation | Phase 1 Interventional | Shanghai Kechow Pharma, Inc. | 3 | |