GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: Melflufen | Pepaxti® | Pepaxto® | Prodrug J 1 | Prodrug J-1
melphalan flufenamide is an approved drug (FDA (2021), EMA (2022))
Compound class:
Synthetic organic
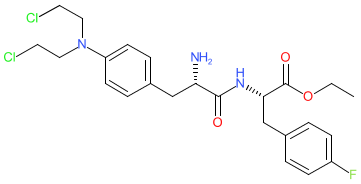
Comment: Melphalan flufenamide is a peptide-conjugated prodrug of the cytotoxic DNA alkylating drug melphalan [3]. It is highly lipophilic and is rapidly transported into cells where it is quickly hydrolysed by aminopeptidases that are overexpressed by some cancer cells. Melphalan flufenamide is being evaluated as a treatment for multiple myeloma [2,5-6].
The IUPAC Condensed peptide sequence for melphalan flufenamide is H-Phe(Unk)-Phe(4-F)-OEt. |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| In February 2021, melphalan flufenamide (in combination with dexamethasone) was granted its first approval by the FDA. It was indicated for the treatment of extensively previously treated relapsed or refractory multiple myeloma [4]. As of July 2021, there were nine melflufen/J-1 clinical studies registered on clinicaltrials.gov. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Once inside aminopeptidase-positive tumour cells, melphalan flufenamide is hydrolysed to release the active alkylating agent melphalan. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03151811 | A Study of Melphalan Flufenamide (Melflufen)-Dex or Pomalidomide-dex for RRMM Patients Refractory to Lenalidomide | Phase 3 Interventional | Oncopeptides AB | ||
| NCT01897714 | Safety and Efficacy of Melflufen and Dexamethasone in Relapsed and/or Relapsed-Refractory Multiple Myeloma Patients | Phase 1/Phase 2 Interventional | Oncopeptides AB | 1 | |