GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
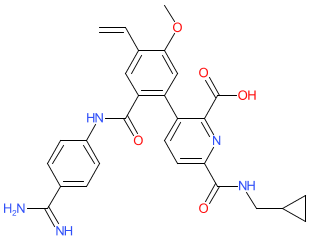
Synonyms: BCX-4161 | BCX4161
Compound class:
Synthetic organic
Comment: Avoralstat (BCX-4161) is a small molecule, oral plasma kallikrein (a serine protease that generates bradykinin from kininogen) inhibitor [3]. Data pubished in 2021 indicate that avoralstat inhibits TMPRSS2 (a protease required for spike protein-mediated SARS-CoV-2 viral entry via ACE2) with comparable inhibitory activity to camostat [2].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| BCX-4161 is/was a clinical candidate for the treatment of hereditary angioedema (HAE) [1]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02125162 | A Study of the Relative Bioavailability of a New Formulation of BCX4161 and the Effect of Food on BCX4161 | Phase 1 Interventional | BioCryst Pharmaceuticals | ||
| NCT02670720 | Open-label, Long-term Safety Study of Avoralstat in Subjects With Hereditary Angioedema | Phase 3 Interventional | BioCryst Pharmaceuticals | This study was terminated as data from a previous efficacy study did not support continued development. | |
| NCT02303626 | 12-Week Safety and Efficacy Study of BCX4161 as an Oral Prophylaxis Against HAE Attacks | Phase 2/Phase 3 Interventional | BioCryst Pharmaceuticals | This study did not demonstrate efficacy of avoralstat as HAE prophylaxis. | 4 |