GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: Genz-682452 | GZ/SAR402671 | ibiglustat | SAR402671
Compound class:
Synthetic organic
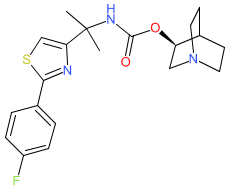
Comment: Venglustat is a clinical stage oral glucosylceramide synthase (GCS) inhibitor. GCS is encoded by the UGCG gene. It was developed for potential to treat diseases in which the glycolipid glucosylceramide accumulates and leads to lysosomal dysfunction and organ damage [3-4]. Venglustat inhibits glucosylceramide synthesis. Its chemical structure is claimed in Genzyme's patent WO2012129084A2 [1]. It has subsequently been reported to act as a substrate-competitive inhibitor of N-terminal Xaa-Pro-Lys N-methyltransferase 1 (NTMT1; Q9BV86; IC50 420 nM) [2].
The venglustat analogue GENZ-667161 and the eliglustat analogue GENZ-123346 have been shown to inhibit replication of SARS-CoV-2 in vitro [5], potentially by disrupting the host cell membrane dynamics which the virions depend upon for infection. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| This clinical candidate has failed to demonstrate efficacy in Parkinson's disease and autosomal dominant polycystic kidney disease (ADPKD). Studies in Gaucher disease type 3 [6], Fabry disease [4] and GM2 gangliosidosis are still ongoing. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT01674036 | Safety, Tolerability and Pharmacokinetics of Genz-682452 in Healthy Men | Phase 1 Interventional | Sanofi | ||
| NCT01710826 | A Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics Study of Genz-682452 in Healthy Volunteers | Phase 1 Interventional | Sanofi | ||
| NCT02906020 | A Global Study to Assess the Drug Dynamics, Efficacy, and Safety of Venglustat (GZ/SAR402671) in Parkinson's Disease Patients Carrying a Glucocerebrosidase (GBA) Gene Mutation | Phase 2 Interventional | Sanofi | ||
| NCT02228460 | Evaluate the Safety, Pharmacodynamics, Pharmacokinetics, and Exploratory Efficacy of GZ/SAR402671 in Treatment-naïve Adult Male Patients With Fabry Disease | Phase 2 Interventional | Sanofi | ||
| NCT02843035 | Venglustat in Combination With Cerezyme in Adult and Pediatric Patients With Gaucher Disease Type 3 | Phase 2/Phase 3 Interventional | Sanofi | ||
| NCT04221451 | A Multinational, Randomized, Double-blind, Placebo-controlled Study to Assess the Efficacy, Pharmacodynamics, Pharmacokinetics, and Safety of Venglustat in Late-onset GM2 | Phase 3 Interventional | Sanofi | ||
| NCT04705051 | Long-term Treatment of Autosomal Dominant Polycystic Kidney Disease (ADPKD) With Venglustat | Phase 3 Interventional | Sanofi | ||