GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: Emovit® | ICI-58,834 | ICI-58834 | Qelbree®
viloxazine is an approved drug (FDA (2021))
Compound class:
Synthetic organic
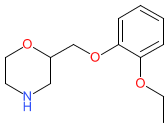
Comment: Viloxazine was originally described as a selective norepinephrine reuptake inhibitor (NRI), principally based on its ability to potentiate noradrenergic effects. It also modulates 5-HT (serotonin) activity, via antagonistic activity at 5-HT2B and agonistic activity at 5-HT2C receptors, and it increases extracellular 5-HT levels in the prefrontal cortex (PFC) [4]. Viloxazine's chemical structure is different from conventional tri- or tetra-cyclic antidepressants. The INN stipulates a racemic mixture of enantiomers. We show the structure without specified stereochemistry to represent the mixture. The hydrocholride salt (SPN-812) was progressed by Supernus Pharmaceuticals for attention deficit hyperactivity disorder (ADHD) in children and adults.
|
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Lamb YN. (2021)
Viloxazine: Pediatric First Approval. Paediatr Drugs, 23 (4): 403-409. [PMID:34036533] |
|
2. Nasser A, Kosheleff AR, Hull JT, Liranso T, Qin P, Busse GD, O'Neal W, Fava M, Faraone SV, Rubin J. (2021)
Translating Attention-Deficit/Hyperactivity Disorder Rating Scale-5 and Weiss Functional Impairment Rating Scale-Parent Effectiveness Scores into Clinical Global Impressions Clinical Significance Levels in Four Randomized Clinical Trials of SPN-812 (Viloxazine Extended-Release) in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. J Child Adolesc Psychopharmacol, 31 (3): 214-226. [PMID:33600233] |
|
3. Nasser A, Liranso T, Adewole T, Fry N, Hull JT, Chowdhry F, Busse GD, Cutler AJ, Jones NJ, Findling RL et al.. (2020)
A Phase III, Randomized, Placebo-controlled Trial to Assess the Efficacy and Safety of Once-daily SPN-812 (Viloxazine Extended-release) in the Treatment of Attention-deficit/Hyperactivity Disorder in School-age Children. Clin Ther, 42 (8): 1452-1466. [PMID:32723670] |
|
4. Yu C, Garcia-Olivares J, Candler S, Schwabe S, Maletic V. (2020)
New Insights into the Mechanism of Action of Viloxazine: Serotonin and Norepinephrine Modulating Properties. J Exp Pharmacol, 12: 285-300. [PMID:32943948] |