GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
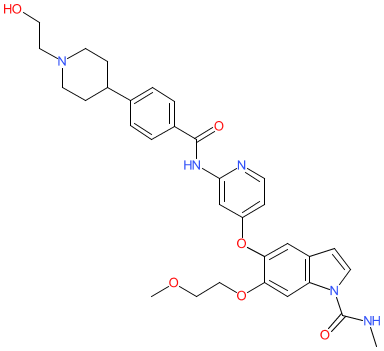
Synonyms: E-7090 | E7090 | Example 22 [WO2014129477A1]
tasurgratinib is an approved drug
Compound class:
Synthetic organic
Comment: The chemical structure for the INN tasurgratinib resolves to E7090 via PubChem, and is claimed as Example 22 in patent WO2014129477A1 as an inhibitor of FGFR pathway signalling [2]. Tasurgratinib (E7090) is an orally active FGFR1-3 inhibitor [6]. kinetic interaction analysis suggests that it is a type V inhibitor (e.g. the EGFR2/3 inhibitor lenvatinib). It was designed for the treatment of solid tumours with oncogenic FGFR gene fusions [3].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| tasurgratinib (as E-7090A) was advanced to clinical evaluations in subjects with advanced solid tumours harbouring FGFR2 fusions, including cholangiocarcinoma.The Japanese drug regulator (PMDA) approved tasurgratinib (Tasfygo®) in September 2024 [4]. It is indicated to treat FGFR2 fusion-positive unresectable, biliary tract cancer. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04493255 | A Study to Determine the Metabolism and Elimination of [14C]E7090 in Healthy Male Participants | Phase 1 Interventional | Eisai Inc. | ||
| NCT04238715 | A Study of E7090 in Participants With Unresectable Advanced or Metastatic Cholangiocarcinoma With Fibroblast Growth Factor Receptor (FGFR) 2 Gene Fusion | Phase 2 Interventional | Eisai Inc. | ||
| NCT04962867 | NCCH2006/MK010 Trial (FORTUNE Trial) | Phase 2 Interventional | National Cancer Center, Japan | 1 | |
| NCT02275910 | Phase 1 Study of E7090 in Subjects With Solid Tumor | Phase 1 Interventional | Eisai Inc. | 5 | |