GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
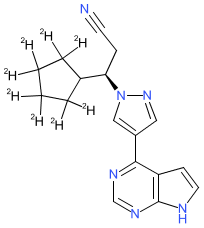
Synonyms: CTP-543 | CTP543 | D8-ruxolitinib | Leqselvi®
deuruxolitinib is an approved drug (FDA (2024))
Compound class:
Synthetic organic
Comment: Deuruxolitinib (CTP-543) is a deuterium-modified analogue of the JAK inhibitor ruxolitinib. It is an oral inhibitor that inhibits JAK1/2. The chemical structure, and its use in hair loss disorders are claimed in patent WO2017192905A1 [3].
|
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Ismail FF, Sinclair R. (2020)
JAK inhibition in the treatment of alopecia areata - a promising new dawn?. Expert Rev Clin Pharmacol, 13 (1): 43-51. [PMID:31865802] |
|
2. King B, Senna MM, Mesinkovska NA, Lynde C, Zirwas M, Maari C, Prajapati VH, Sapra S, Brzewski P, Osman L et al.. (2024)
Efficacy and safety of deuruxolitinib, an oral selective Janus kinase inhibitor, in adults with alopecia areata: Results from the Phase 3 randomized, controlled trial (THRIVE-AA1). J Am Acad Dermatol, 91 (5): 880-888. [PMID:39053611] |
|
3. Wagner AT, Cassella JV, Graham PB, Braman V, Uttamsingh V, Von Hehn J, Hamilton CE. (2017)
Treatment of hair loss disorders with deuterated jak inhibitors. Patent number: WO2017192905A1. Assignee: Concert Pharmaceuticals. Priority date: 04/05/2016. Publication date: 09/11/2017. |