GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
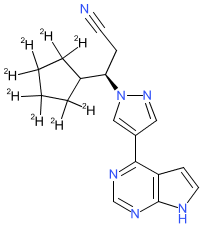
Synonyms: CTP-543 | CTP543 | D8-ruxolitinib | Leqselvi®
deuruxolitinib is an approved drug (FDA (2024))
Compound class:
Synthetic organic
Comment: Deuruxolitinib (CTP-543) is a deuterium-modified analogue of the JAK inhibitor ruxolitinib. It is an oral inhibitor that inhibits JAK1/2. The chemical structure, and its use in hair loss disorders are claimed in patent WO2017192905A1 [3].
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Deuruxolitinib (CTP-543) was progressed through clinical development as a treatment for alopecia areata [1]. The FDA granted Breakthrough Therapy and Fast Track designations for CTP-543. Full FDA approval was granted in July 2024, for the treament of severe alopecia areata, based on efficacy data from the THRIVE-AA1 and THRIVE-AA2 studies. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04518995 | A Phase 3 Study to Evaluate the Efficacy and Safety of CTP-543 in Adult Patients With Moderate to Severe Alopecia Areata | Phase 3 Interventional | Concert Pharmaceuticals | THRIVE-AA1 study | 2 |
| NCT03137381 | Study to Evaluate the Safety and Efficacy of CTP-543 in Adult Patients With Moderate to Severe Alopecia Areata | Phase 2 Interventional | Concert Pharmaceuticals | ||
| NCT04797650 | Study to Evaluate the Efficacy and Safety of CTP-543 in Adults With Moderate to Severe Alopecia Areata (THRIVE-AA2) | Phase 3 Interventional | Concert Pharmaceuticals | THRIVE-AA2 study | 2 |