GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
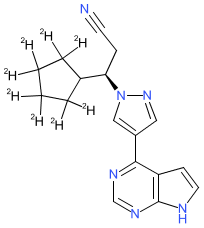
Synonyms: CTP-543 | CTP543 | D8-ruxolitinib | Leqselvi®
deuruxolitinib is an approved drug (FDA (2024))
Compound class:
Synthetic organic
Comment: Deuruxolitinib (CTP-543) is a deuterium-modified analogue of the JAK inhibitor ruxolitinib. It is an oral inhibitor that inhibits JAK1/2. The chemical structure, and its use in hair loss disorders are claimed in patent WO2017192905A1 [3].
|
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| We have been unable to find quantitiative potency data for this compound at its intended molecular targets, but this is unlikely to be significantly different from the parent drug ruxolitinib. |
| Selectivity at enzymes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||