GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: AC 2993 | AC002993 | AC2993A | Bydureon® | Byetta®
exendin-4 is an approved drug (FDA (2005), EMA (2006))
Compound class:
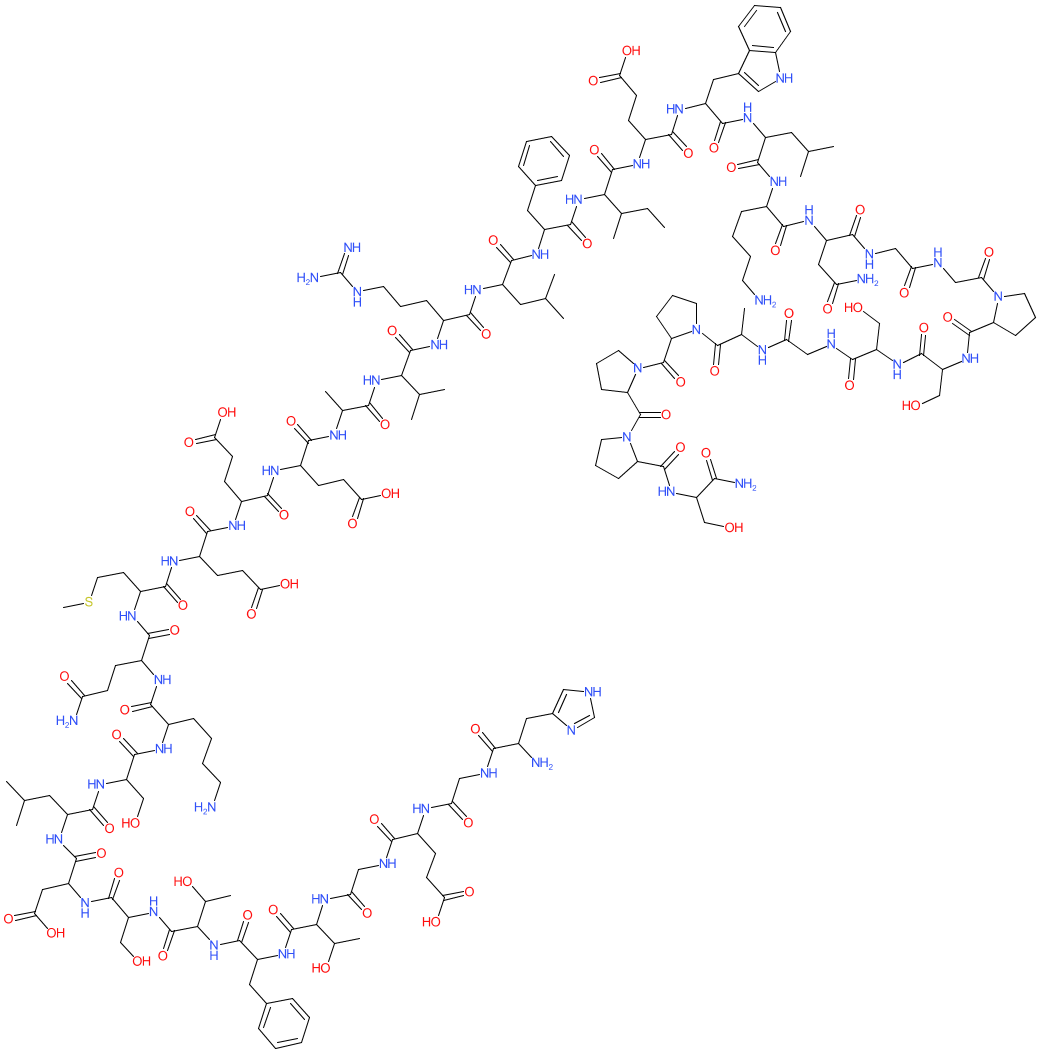
Peptide
Comment: This peptide is found in the venom of the Gila monster Heloderma suspectum. Exenatide is the synthetic form of the peptide used in clinical practice. Exendin-4 is a GLP-1 analogue/mimetic [3]. There is some ambiguity surrounding the exact stereochemistry of exendin-4. Representations of the peptide's full chemical structure can be accessed using the PubChem, ChEMBL and ChEBI links that we provide in the Database Links table.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖
View more information in the IUPHAR Pharmacology Education Project: exenatide |
|
|||||||||||||||||
| References |
|
1. Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, Hibbert S, Budnik N, Zampedri L, Dickson J et al.. (2017)
Exenatide once weekly versus placebo in Parkinson's disease: a randomised, double-blind, placebo-controlled trial. Lancet, 390 (10103): 1664-1675. [PMID:28781108] |
|
2. Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, Whitton P, Wyse R, Isaacs T, Lees A et al.. (2013)
Exenatide and the treatment of patients with Parkinson's disease. J Clin Invest, 123 (6): 2730-6. [PMID:23728174] |
|
3. Bond A. (2006)
Exenatide (Byetta) as a novel treatment option for type 2 diabetes mellitus. Proc (Bayl Univ Med Cent), 19 (3): 281-4. [PMID:17252050] |
|
4. Jorgensen R, Martini L, Schwartz TW, Elling CE. (2005)
Characterization of glucagon-like peptide-1 receptor beta-arrestin 2 interaction: a high-affinity receptor phenotype. Mol Endocrinol, 19 (3): 812-23. [PMID:15528268] |
|
5. Meissner WG, Remy P, Giordana C, Maltête D, Derkinderen P, Houéto JL, Anheim M, Benatru I, Boraud T, Brefel-Courbon C et al.. (2024)
Trial of Lixisenatide in Early Parkinson's Disease. N Engl J Med, 390 (13): 1176-1185. [PMID:38598572] |
|
6. Miranda LP, Winters KA, Gegg CV, Patel A, Aral J, Long J, Zhang J, Diamond S, Guido M, Stanislaus S et al.. (2008)
Design and synthesis of conformationally constrained glucagon-like peptide-1 derivatives with increased plasma stability and prolonged in vivo activity. J Med Chem, 51 (9): 2758-65. [PMID:18412318] |
|
7. Pagano G, Taylor KI, Anzures Cabrera J, Simuni T, Marek K, Postuma RB, Pavese N, Stocchi F, Brockmann K, Svoboda H et al.. (2024)
Prasinezumab slows motor progression in rapidly progressing early-stage Parkinson's disease. Nat Med, 30 (4): 1096-1103. [PMID:38622249] |