GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: AT-527 | AT527 | RO-7496998 | RO7496998
Compound class:
Synthetic organic
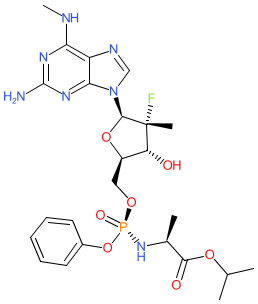
Comment: AT-527 is the orally bioavailable hemi-sulfate salt of AT-511, which is a direct-acting antiviral compound that disrupts activity of the viral RNA polymerase [1]. It was originally developed to combat hepatitis C (HCV) infection, but has been redeployed for SARS-CoV-2. Chemically AT-527 is a phosphoramidate purine nucleotide and it is metabolised to a biologically active guanosine triphosphate analogue in vivo. Preclinical results showing pan-genotype activity against HCV were published in early 2020 [2]. In October 2020 anti-SARS-CoV-2 activity was reported in preprint format [3], which converted to full peer reviewed publication in March 2021 [4].
AT-527 was developed by Atea Pharmaceuticals [5]. In October 2020 it was announced that Roche were purchasing the rights to AT-527 (and gave it the research code RO7496998) outside of the US. In the same press release, it was revealed that AT-527 would be evaluated in Phase 3 trial in early 2021 to determine its efficacy as an early treatment for COVID-19 in non-hospitalised patients. This is a complex molecule with 5 stereo-centres, so there are several CIDs with the same connectivity in PubChem. We chose to represent the molecule from CID 122527275 as it is linked to a FDA UNique Ingredient Identifier (UNII) code. This CID represents the parent molecule. For the hemi-sulfate salt structure see PubChem CID 155926085. |
|
|||||||||||||||||||||||||||||||||||
Classification  |
|
| Compound class | Synthetic organic |
International Nonproprietary Names  |
|
| INN number | INN |
| 12031 | bemnifosbuvir |
Synonyms  |
| AT-527 | AT527 | RO-7496998 | RO7496998 |
Database Links  |
|
| CAS Registry No. | 1998705-64-8 (source: WHO INN record) |
| GtoPdb PubChem SID | 434321730 |
| PubChem CID | 122527275 |
| Search Google for chemical match using the InChIKey | OISLSHLAXHALQZ-LZEIJKKFSA-N |
| Search Google for chemicals with the same backbone | OISLSHLAXHALQZ |
| Search PubMed clinical trials | bemnifosbuvir |
| Search PubMed titles | bemnifosbuvir |
| Search PubMed titles/abstracts | bemnifosbuvir |
| UniChem Compound Search for chemical match using the InChIKey | OISLSHLAXHALQZ-LZEIJKKFSA-N |
| UniChem Connectivity Search for chemical match using the InChIKey | OISLSHLAXHALQZ-LZEIJKKFSA-N |
Product suppliers
View disclaimer
Sponsored products disclaimer
Product supplier links are provided as a service to assist in identifying commercial suppliers of reagents that are mentioned on the IUPHAR/BPS Guide to PHARMACOLOGY database website, and do not imply their endorsement by NC-IUPHAR.
Links are provided in return for sponsorship, used to fund improvements to this database. The sponsorship account is managed and audited by the University of Edinburgh, a charitable body registered in Scotland, SC005336. If you are interested in sponsoring the database, please contact us.
✖
Sponsored products disclaimer
Product supplier links are provided as a service to assist in identifying commercial suppliers of reagents that are mentioned on the IUPHAR/BPS Guide to PHARMACOLOGY database website, and do not imply their endorsement by NC-IUPHAR.
Links are provided in return for sponsorship, used to fund improvements to this database. The sponsorship account is managed and audited by the University of Edinburgh, a charitable body registered in Scotland, SC005336. If you are interested in sponsoring the database, please contact us.
✖|
Bemnifosbuvir (links to external site)
Cat. No. HY-137958A |