GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
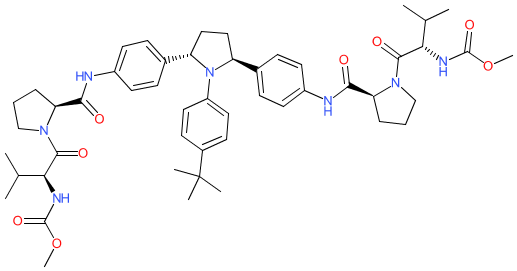
Synonyms: ABT-267 | ABT267 | compound 38 [PMID: 24400777] | Technivie® (ombitasvir + paritaprevir + ritonavir) | Viekirax® (ombitasvir + paritaprevir + ritonavir; EU)
ombitasvir is an approved drug (FDA (2015), EMA (2020))
Compound class:
Synthetic organic
Comment: Ombitasvir is an orally available, direct-acting inhibitor of the hepatitis C virus (HCV) non-structural protein 5A (NS5A) replication complex [1]. In common with many other NS5A inhibitors it is a dinucleotide derivative.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Ombitasvir is used clinically in fixed-dose combination with paritaprevir and ritonavir to treat chronic HCV infection. The Technivie® brand has been discontinued in the USA. |
External links  |
|
For extended ADME data see the following: Drugs.com European Medicines Agency (EMA) |