GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
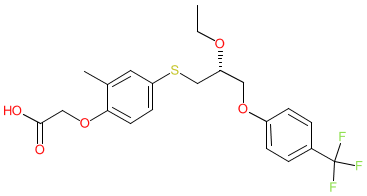
Synonyms: compound 3r [PMID: 17524639] | Livdelzi® | MBX-8025 | MBX8025
seladelpar is an approved drug (FDA (2024))
Compound class:
Synthetic organic
Comment: Seladelpar (MBX-8025) is a selective PPARδ agonist [4] that was initially developed as a therapy for atherogenic dyslipidemia and nonalcoholic steatohepatitis (NASH).
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Phase 2 studies were suspended when histological tests detected signs of liver damage in some NASH trial subjects. However, other measures of liver injury (e.g. liver enzyme levels) did not point to liver damage. Following a review, and lifting of the FDA's clinical hold for all three seladelpar indications, the drug's developer re-initiated studies (in July 2020). Following positive results, the FDA approved seladelpar to treat primary biliary cholangitis in addition to or as an alternative to ursodeoxycholic acid, in August 2024 [2]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02609048 | Study to Evaluate the Effects of Two Doses of MBX-8025 in Subjects With Primary Biliary Cirrhosis (PBC) | Phase 2 Interventional | CymaBay Therapeutics, Inc. | 3 | |
| NCT00701883 | Safety and Benefit of MBX-8025 With and Without Commonly Used Statins in Moderately Overweight Patients With High Cholesterol | Phase 2 Interventional | CymaBay Therapeutics, Inc. | 1 | |
| NCT03301506 | Seladelpar in Subjects With Primary Biliary Cholangitis (PBC) | Phase 2/Phase 3 Interventional | CymaBay Therapeutics, Inc. | ||