GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Natural product
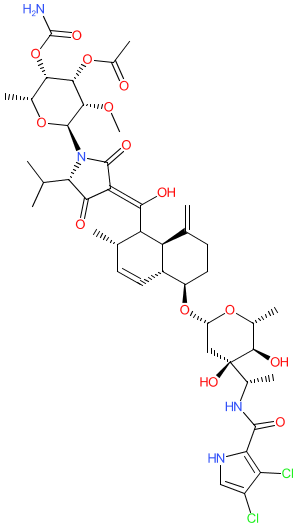
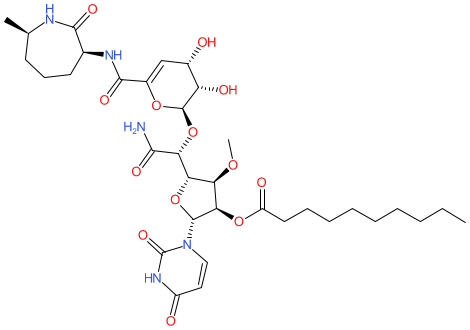
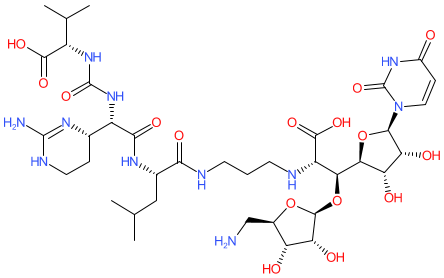
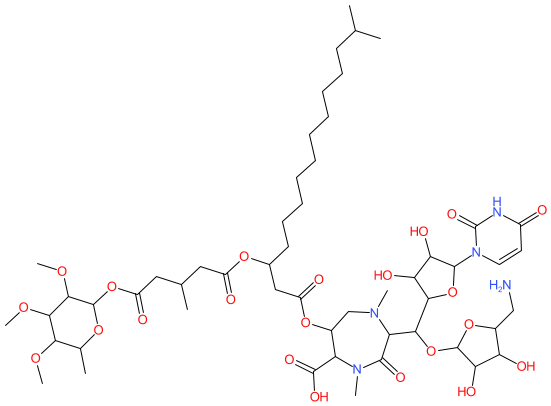
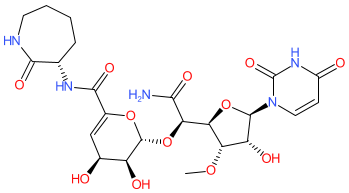
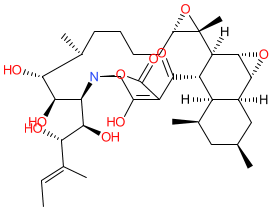
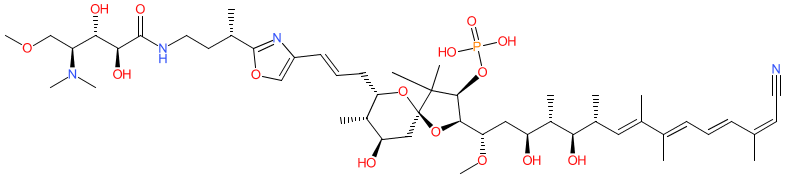
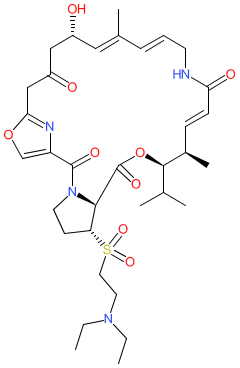
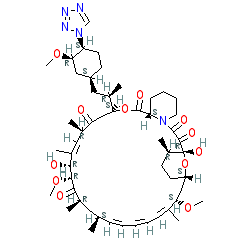
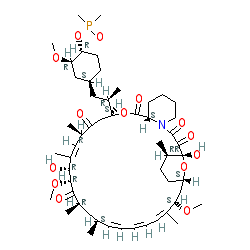
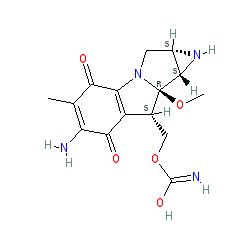
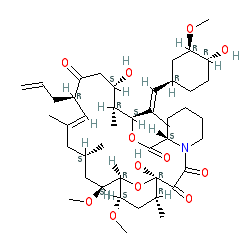
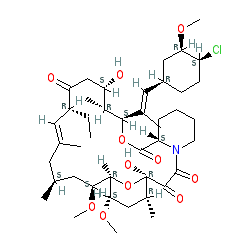
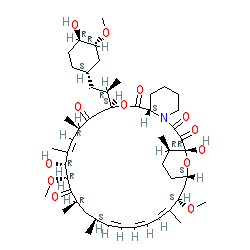
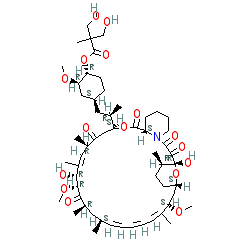
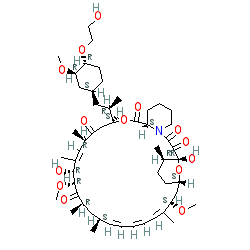
Comment: Kibdelomycin is a broad-spectrum bactericidal agent with activity against aerobic bacteria [1]. It inhibits bacterial growth by inhibiting the bacterial DNA replication enzymes DNA gyrase and topoisomerase IV.
|
|
|||||||||||||||||||||||||||||||||||
For advanced searching click here to open chemical structure editor